Safeguarding standards and strengthening science
At BMJ Group, we set the benchmark for publishing integrity, shaping best practices across the industry and supporting the research community to publish responsibly.
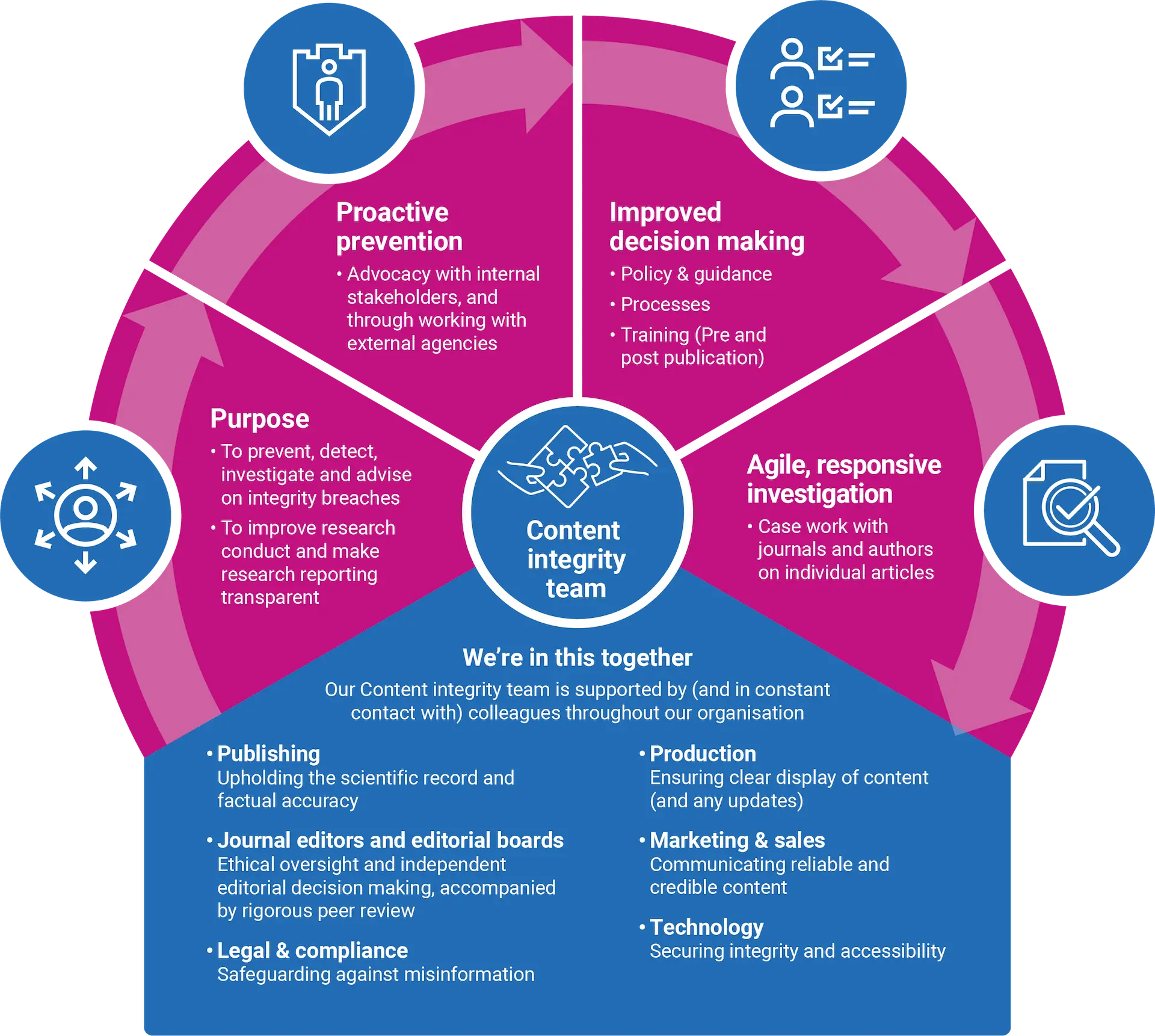
Content integrity underpins everything we publish. Our dedicated team works to prevent, identify and address significant errors and instances of scientific misconduct or malpractice in the content published across all our journals.
For authors and organisations, this means a fair, transparent publishing process, reputation protection, and greater confidence that their research will be respected, cited, and used to drive real world change.
Our people

Dr Helen Macdonald
BA MBBS MSc nMRCGP
Publications ethics and content integrity editor
At BMJ Group since 2008, Helen has led on ethics, research and education, shaping initiatives such as BMJ Rapid Recommendations, Better evidence and Too much medicine. A qualified GP, she brings clinical insight and academic rigour to uphold integrity and clarity across the Group, supporting ethical decision making and strong research practice. Learn more about Helen Macdonald.

Helen Beynon
MA Oxon
Research integrity manager and COPE adviser
Responsible for ethical and legal issues across editorial and production, Helen resolves cases in line with policy and advances publishing standards globally. She represents BMJ Group in industry forums and discussions, supporting integrity initiatives across publishing. Previously at Cambridge University Press & Assessment, she began her career at SAGE and holds an MA in Medieval and Modern Languages from the University of Oxford. Learn more about Helen Beynon.
Upholding trust, every step of the way
Integrity underpins everything we publish. Through rigorous editorial standards, transparent policies, and active collaboration across the global publishing community, BMJ Group strengthens trust in medical research.
Our content integrity team ensures every article meets the highest ethical and scientific standards, so clinicians, researchers, and policymakers can act on our content with confidence.
BMJ Group sets the benchmark for publishing integrity, shaping best practice across the industry and supporting the research community to publish responsibly.
Hear it from us
Our approach to content integrity
Why does integrity matter so much? Because every clinical decision depends on trustworthy evidence. In this video, we explain how we prevent bias, protect the scholarly record, and act when concerns arise.
From robust policies to transparent corrections and retractions, we show what integrity looks like in practice, and why it underpins better care.
How we handle the most difficult challenges
Some integrity issues are straightforward. Others aren’t. Here, we walk through how we handle tough cases, questionable images, duplicate submissions, undisclosed relationships, and legal concerns.
You’ll see how we assess evidence, consult experts, communicate with authors, and document decisions, always aligning with COPE guidance and our policies.
BMJ ethics committee
Launched in 2000, BMJ’s Ethics Committee provides expert advice on ethical issues in editorial decision making. Its members bring deep expertise spanning medicine, research, law, bioethics, journalism, and medical editing, ensuring a broad perspective on the toughest ethical questions.
The committee assists the BMJ Group content integrity team with reviewing and developing editorial policies on the most pressing integrity issues and advises editors on complex ethical questions that arise during routine editorial work and research integrity investigations. These include author disputes and consent issues to suspected research misconduct.
Lessons from the Wakefield case
The importance of strong editorial safeguards was underlined by the Andrew Wakefield MMR vaccine case. Initially published in The Lancet in 1998, the paper falsely claimed a link between the MMR vaccine and autism. Years later, The BMJ exposed the research as fraudulent through a landmark investigation by journalist Brian Deer. His reporting revealed serious data misrepresentation and undisclosed conflicts of interest, leading to the paper’s retraction and Wakefield being struck off the medical register.
This case is a powerful reminder of the consequences of weak integrity systems. It helped develop our current ethics and content integrity structures, including the Ethics Committee, to ensure potential misconduct is identified, investigated, and addressed with rigour and transparency.
Working with global integrity partners
As a member of key publishing integrity organisations, BMJ closely follows and has helped develop best practice guidelines, helping to drive and support improved practices. Our Content Integrity team contributes to a number of cross-publisher working groups united by these common goals
COPE: Committee on Publication Ethics
International Committee of Medical Journal Editors (ICMJE)
WAME (World Association of Medical Editors)
Crossref and Crossmark
CLOCKSS/LOCKSS
STM Integrity Hub
Editor roles and responsibilities
BMJ Journals are published with full editorial independence, in line with guidance from the World Association of Medical Editors, COPE, International Committee of Medical Journal Editors, and EQUATOR. Editors are supported to make decisions free from commercial influence and are encouraged to publish evidence based, sometimes controversial views.

